Understanding nematode depth and distribution in bentgrass greens
Plant-parasitic nematodes (PPNs) pose a significant risk to the health and visual quality of golf putting greens. Modified sand-based root zones provide a large pore space environment amenable to nematode movement and population growth, and feeding damage is more quickly realized on golf greens already under intense stress from low mowing and aggressive maintenance practices.
Control of plant-parasitic nematodes (PPNs) on putting greens with nematicides may depend on the seasonal occurrence and depth distribution of target PPN populations. Many nematicide active ingredients have low water solubility and high organic matter affinity, leaving them stuck in the thatch layer (7 and 21). As a result of this limited soil mobility, control of a target PPN deeper in the soil profile is unlikely.
As a further complication, PPN species vary in their lifestyle and can be present outside or inside the root. Determining if and when PPN populations aggregate to a targetable depth may help combat limitations in nematicide efficacy.
This study aimed to determine if plant-parasitic nematode populations on golf course putting greens in Missouri and Indiana peaked at a targetable depth at a specific time in the year, focusing primarily on lance (Hoplolaimus spp.) and root-knot (Meloidogyne spp.) nematodes.
Root-knot nematodes are the most economically damaging PPN due to their explosive and destructive endoparasitic reproductive process and ability to produce new progeny quickly (24). The morphology of root-knot nematodes is dynamic through their life cycle, with gender-specific characteristics that develop after three to eight weeks of plant parasitism (3). However, root-knot nematodes are typically easily recognized due to the large size of females and host specificity between species (3).
Lance nematodes attack a range of cereal crops and turfgrasses and feed both externally (ectoparasitism) and while burrowing through root tissue (endoparasitism) (4,10,17 and 19). Hoplolaimus galeatus is the main species of lance nematode reported to parasitize turfgrasses in the United States (8, 10 and 19). Alternatively, H. stephanus has seldom been reported parasitizing bentgrass outside the East Coast, Georgia and on two golf course greens in the Midwestern United States (11 and 12). Although H. galeatus is a well-documented PPN on turfgrasses in the Southern region, similarities in morphological characteristics (9, 20 and 22) and host range (14) of Hoplolaimus spp. may have resulted in the over-representation of this species.
Effectively timing management strategies, particularly nematicide applications, require knowledge of the biology and seasonal occurrence of PPN populations. Nematicide applications timed when PPNs are shallow in the soil profile and just before a population spike would improve control (5). This study serves as a benchmark to enhance nematicide control by determining the monthly vertical distribution of PPNs on putting greens in Missouri and Indiana. This study also aimed to assess lance and root-knot nematode species on golf greens in the Midwestern United States through DNA sequencing and scanning electron microscopy.
Materials and Methods
In 2021 and 2022, 20 golf putting greens with creeping bentgrass or a mix of creeping bentgrass and Poa annua were sampled. In 2021, ten greens from Missouri and Kansas City (Mo./Kan.) were selected based on prior high lance nematode populations. In 2022, 10 Indiana greens were sampled without a priori knowledge of their potential nematode levels. Sampling occurred in April, June, August and October. Twelve sampling points per green were collected using a 1.9 cm soil probe to a 25 cm depth, stratified into 5 cm increments and aggregated to 100 cm³ of soil per depth.
Samples were processed using a root washer and sucrose-flotation method, with nematodes identified morphologically and counted using a hemocytometer under 40x–400x magnification. Individual PPNs were identified to genus based on general morphological characteristics with a pictorial key to genera.
Nematode populations were statistically analyzed using PROC GLIMMIX in SAS 9.4 to assess vertical distribution and seasonal density changes. Variables included depth, month and their interaction, with the site treated as random. Populations from Mo./Kan. and Indiana were analyzed separately, with means compared using Fisher’s LSD.
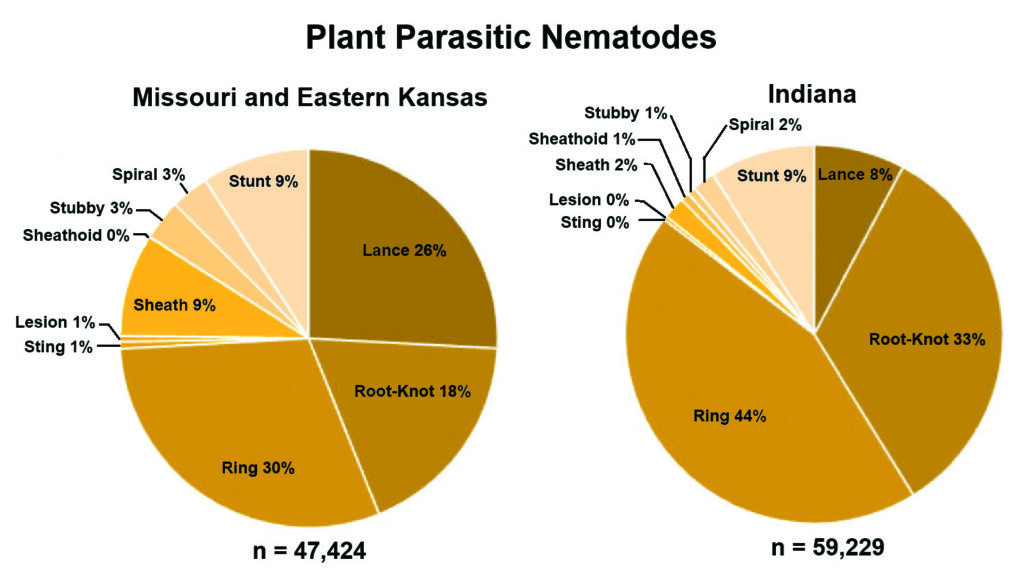
[Figure 1] Distribution of plant-parasitic nematode species sampled from creeping bentgrass putting greens in Missouri and Eastern Kansas in 2021 and Indiana in 2022 in two independent pie charts. Samples were collected during April, June, August and October of 2021 and 2022, respectively. The “n” indicates the total PPNs represented within each chart.

Results
Plant parasitic nematode (PPN) populations were more abundant in Indiana in 2022 than in Mo./Kan. in 2021 (Figure 1). The reduced number of nematicide applications in Indiana sampling locations compared to Mo./Kan. may have caused this difference.
Higher lance nematode populations were found in Mo./Kan. than in Indiana (average of 1,225 and 462, respectively), In 2021, the lance nematode population had a significant depth-by-month interaction in Mo./Kan. (Table 1 and Figure 2). Lance populations sampled across Indiana in 2022 showed no significant depth-by-month interaction. Populations sampled from the upper 0–5 cm of the soil in October 2021 in Missouri were significantly higher than any other depth and month (Table 1 and Figure 2). Lance nematode populations were higher in August 2022 in Indiana than in the other three months sampled (Table 1 and Figure 3).
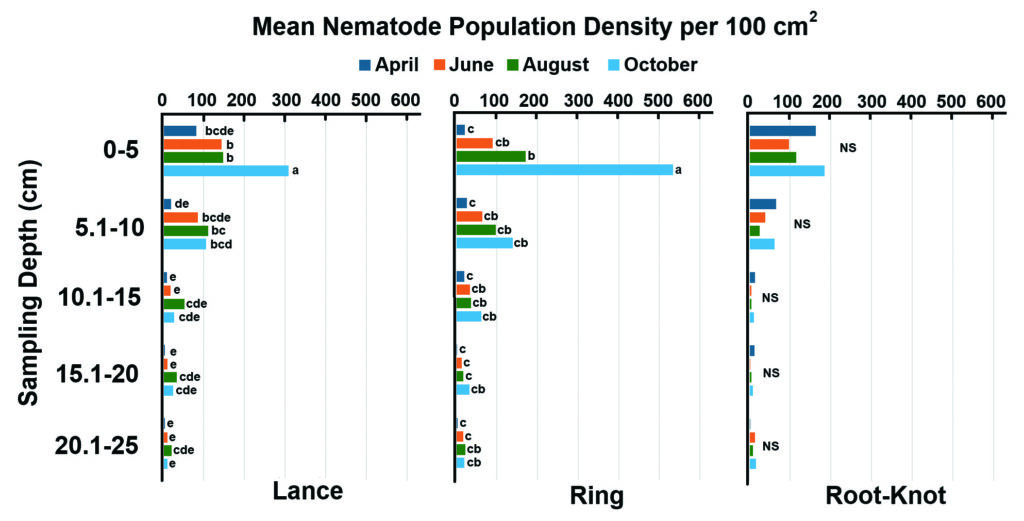
[Figure 2] Missouri and Eastern Kansas 2021 total lance, ring and root-knot nematode population densities by sampling depth and month with soil samples aggregated (100 cm³). Letters indicate significant differences between sampling depths by month analyzed within that individual species (P < 0.05). The “NS” indicates no significant depth-by-month interaction.
Populations of root-knot nematodes in Mo./Kan. were not significantly different between sampling months (Table 1 and Figure 2). In contrast, root-knot nematode population levels in Indiana were significantly higher in April 2022 than in any other month in 2022 (Table 1 and Figure 3). In both states, root-knot nematode populations were higher at the 0–5 cm sampling depth in 2021 than all other depths, and the 5.1–10 cm depth had higher populations than deeper samples (Table 1, Figures 2 and 3).
The most abundant PPN in Mo./Kan. and Indiana was the ring nematode (Criconemoides spp.) (Figure 1). In Mo./Kan., ring and free-living nematodes were significantly more abundant in the 0–5 cm range during October than any other depth–month combination (Figure 2). In Indiana, higher ring nematode populations occurred during August at a 0–5 cm depth than any other depth–month combination (Figure 3).
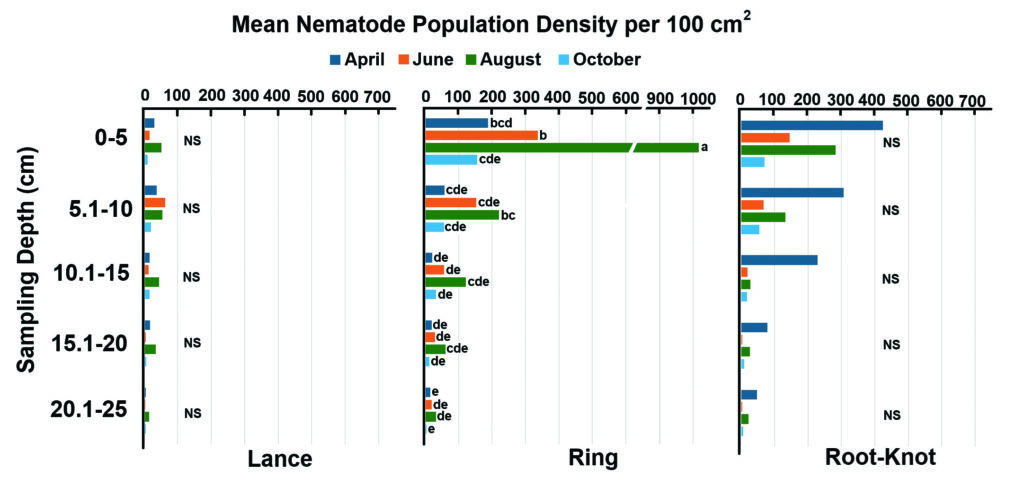
[Figure 3] Indiana 2022 total lance, ring and root-knot nematode population densities by sampling depth and month with soil samples aggregated (100 cm³). Letters indicate significant differences between sampling depths by month analyzed within that individual species (P < 0.05). The “NS” indicates no significant depth-by-month interaction.
Discussion
Populations of lance, root-knot and ring nematodes varied seasonally across all sampling sites and depths, a finding consistent with prior research. While some studies reported similar trends across cropping systems, others highlighted significant differences (1, 6, 13, 16, 18 and 19). Aggregated distributions within greens can result in vastly different population densities, even a few meters apart (19). The findings highlight the limitations of current sampling methods and the difficulty in extrapolating overall PPN populations.
In turfgrass, lance nematode populations are influenced by soil temperature and reproductive cycles, although some studies indicate no association (13, 19 and 23). Declining root biomass creates a feedback loop limiting PPN population density due to increased resource competition (6). Evidence also suggests lance nematodes may shift to endoparasitism when soil temperatures reach specific thresholds, a factor not accounted for in this study (19). As expected, lance nematode populations at several sites in Mo./Kan. with a history of them on the site were above the treatment threshold, particularly in the October sampling period. Conversely, lance nematodes were more rare, at lower populations and below nematicide application thresholds in Indiana.
Hoplolaimus stephanus was the most prevalent lance nematode on Midwest bentgrass greens but remains underreported in literature. Studies may misidentify the species as H. galeatus, which predominates in warmer regions and on different turfgrass species (14). This study reinforces the underrepresentation of H. stephanus in research and emphasizes the potential for geographic and host factors to shape nematode distribution (11, 14, 20 and 23). Additionally, practices such as topdressing with contaminated sand, golf equipment transfer or proximity to agricultural systems may introduce nematodes to putting greens over time (15).
Root-knot nematode populations found in this study were generally below nematicide treatment thresholds (33). In Indiana, they were more highly concentrated at the 0–5 cm depth in April, consistent with some studies but contrasting others that observed late fall peaks (1, 2, 18 and 19). Discrepancies may arise from differences in sampling periods or specific host-crop interactions. For example, early-year moisture levels may induce egg hatching, leading to population fluctuations over time.
Similarly, this study found ring nematodes below nematicide treatment thresholds. Ring nematodes displayed seasonal and depth-related interactions, with population peaks varying by region. Indiana populations peaked in August, while Mo./Kan. peaked in October. Previous studies revealed inconsistent year-to-year trends, even within the same golf course, further complicating predictions (2). Population dynamics often differed across greens within a course, discrediting attempts to generalize findings (23).
Conclusion
This study underscores the importance of regionally characterizing nematode species to improve management strategies. Spring-applied nematicides could coincide with population spikes and maintain acceptable levels through the summer stress period. However, the residual efficacy of nematicides, such as abamectin (short half-life) and fluopyram (longer half-life), is a critical factor in designing a program aimed at season-long control. Additional fall applications may target fall population peaks more aptly, particularly in Mo./Kan., where lance nematodes were more abundant in October.
Research Takeaways
- Overall, plat parasitic nematode (PPN) populations were most abundant in Indiana (2022) than in Missouri/Kansas (2021), possibly due to the lack of nematicide applications at IN sampling sites. Lance nematodes were targeted and more prevalent at Mo./Kan. sites, while root-knot and ring nematodes were more abundant in Indiana sites.
- In October, lance nematodes populations in Mo./Kan. (2021) peaked in the 0-5 cm spoil depth, showing significant depth-by-month interactions.
- Root-knot nematode populations in Mo./Kan. (2021) were consistent across months, with the highest density at 0-5 cm depth. Indiana populations peaked in April with higher densities in the upper soil layers (0-10 cm).
- Ring nematodes were the most abundant parasitic nematodes found in both regions, with higher populations in Indiana sites. Ring nematodes however, do not cause as much feeding damage as other species and are seldom above any action threshold.
The article was adapted from the following source: McCurdy, Asa L.; Barizon, Jefferson; and Miller, G.L. 2024. Depth distribution of plant-parasitic nematodes on bentgrass golf greens in Missouri and Indiana. Journal of Nematology. 56:1-15. DOI: 10.2478/jofnem-2024-0006
For additional information, contact Lee Miller, Ph.D. Purdue University, Department of Botany and Plant Pathology, turfpath@purdue.edu.
Related Articles
Examines nematodes as a form of pest control on the course
Researchers examine nematodes and their control
References
1. Barker KR, Nusbaum CJ, Nelson LA. Seasonal population dynamics of selected plant-parasitic nematodes as measured by three extraction procedures. Journal of Nematology. 1969;1:232–239.
2. Davis R, Kane R, Wilkinson H, Noel G. Population fluctuations of three nematode genera in putting greens in northern Illinois. Journal of Nematology. 1994;26:522–530.
3. Eisenback J, Sasser J, Carter C. Diagnostic characters useful in the identification of the four most common species of root-knot nematodes (Meloidogyne spp.). An advanced treatise on Meloidogyne. 1985;1:95–112.
4. Fassuliotis G. Host range of the Columbia lance nematode, Hoplolaimus columbus [Economic plants]. Plant Disease Reporter. 1974;58:1000–1002.
5. Ferris H. Nematode economic thresholds: Derivation, requirements, and theoretical considerations. Journal of Nematology. 1978;10:341–350.
6. Ferris H. Density-dependent nematode seasonal multiplication rates and overwinter survivorship: A critical point model. Journal of Nematology. 1985;17:93–100.
7. Gannon TW, Jeffries MD, Ahmed KA. Irrigation and soil surfactants affect abamectin distribution in soil. Crop Science. 2017;57:573–580.
8. Giblin-Davis RM, McDaniel LL, Bilz FG. Isolates of the Pasteuria penetrans group from phytoparasitic nematodes in bermudagrass turf. Journal of Nematology. 1990;22:750–762.
9. Handoo ZA, Golden AM. A key and diagnostic compendium to the species of the genus Hoplolaimus Daday, 1905 (Nematoda: Hoplolaimidae). Journal of Nematology. 1992;24:45–53.
10. Henn R, Dunn R. Reproduction of Hoplolaimus galeatus and growth of seven St. Augustinegrass (Stenotaphrum secundatum) cultivars. Nematropica. 1989;6:81–87.
11. Holguin CM, Baeza JA, Mueller JD, Agudelo, P. High genetic diversity and geographic subdivision of three lance nematode species (Hoplolaimus spp.) in the United States. Ecology and Evolution. 2015;14:2929–2944.
12. Jagdale GB, Nugraha GT, Martin K, Martinez- Espinoza AD, Hajihassani A. Occurrence of the lance nematode Hoplolaimus stephanus infecting bentgrass Agrostis stolonifera in Georgia, USA. Plant Health Progress. 2022;23:162–165.
13. Lucas L, Barker K, Blake C. Seasonal changes in nematode densities on bentgrass golf greens in North Carolina. Plant disease reporter. 1978;62:373–376.
14. Ma X, Agudelo P, Mueller JD, Knap HT. Molecular characterization and phylogenetic analysis of Hoplolaimus tephanus. Journal of Nematology. 2011;43:25–34.
15. McClure MA, Nischwitz C, Skantar AM, Schmitt ME, Subbotin SA. Root-knot nematodes in golf course greens of the western United States. Plant Disease. 2012;96:635–647.
16. O’Bannon J, Radewald J, Tomerlin A. Population fluctuation of three parasitic nematodes in Florida citrus. Journal of Nematology. 1972;4:194–199.
17. Robbins R, Riggs R, Von Steen D. Results of annual phytoparasitic nematode surveys of Arkansas soybean fields, 1978-1986. Journal of Nematology. 1987;19:50–55.
18. Sasser J, Nusbaum C. Seasonal fluctuations and host specificity of root-knot nematode populations in 2-year tobacco rotation plots. Phytopathology. 1955;45:540–545.
19. Settle D, Fry J, Todd T, Tisserat N. Population dynamics of the lance nematode (Hoplolaimus galeatus) in creeping bentgrass. Plant Disease. 2006;90:44–50.
20. Sher S. Revision of the Hoplolaiminae (Nematoda). II. Hoplolaimes Daday, 1905 and Aorolaimus n. gen. Nematologica. 1963;9:267–295.
21. USEPA. Environmental fate and ground water branch review of abamectin. USEPA Rep. D187271. Washington: U.S. Gov. Print Office; 1993.
22. Vovlas N, Castillo P, Barcina AG. SEM observations on two species of Hoplolaimus daday, 1905 (Nematoda: Hoplolaimidae). Nematologia Mediterranea. 1991;15: 305–309.
23. Wick R, Vittum P, Swier S. Spatial and temporal distribution of plant parasitic nematodes in putting greens in the New England region. Phytopathology. 1988;78:1521(Abstr.).
24. Wick RL. Population dynamics of nematodes in putting greens. Golf Course Management. 1989;57: 100–112.
25. Stowell, L., and Gelernter W. 2008. Evaluation of nematode thresholds and turf damage. http://www.paceturf.org/index.php/journal/evaluation_of_nematode_thresholds_and_turf_damage/