Nematodes in southern turfgrasses
Nematodes have been recognized as significant pests of turfgrasses in the South for more than 60 years, corresponding with increased U.S. economic vitality after World War II and heightened interest in golf. That heightened interest added dollars to promote better playing conditions and led scientists to investigate nematodes as a potential cause of stressed turf. Florida was the first state to report nematode injury in bermudagrass (Cynodon dactylon) turf.
After this initial recognition, many U.S. locations reported nematodes associated with damaged turf. In the ensuing decades, many products were evaluated for nematode control. Initial reports usually were surveys of the genera and species of nematodes associated with various turfgrasses, and included familiar nematodes such as Belonolaimus longicaudatus (sting), Hoplolaimus galeatus (lance), Trichodorus and Paratrichodorus spp. (stubby root) and others such as Helicotylenchus (spiral), Criconemella (ring), Criconemoides (sheathoid), Hemicycliophora (sheath), Tylenchorrhynchus (stunt), Meloidogyne (root-knot) and Hypsoperine (false root-knot nematode). Surveys still are important and they continue, however, identification now employs morphology and molecular identification tools that allow better identification.
A recent survey of North Carolina and South Carolina golf courses revealed a high diversity of 24 nematode species belonging to 19 genera and 11 families. Of those, 23 species were found in South Carolina, 19 species in North Carolina and 18 species in both states. Helicotylenchus dihystera, Mesocriconema xenoplax, Hoplolaimus galeatus, Tylenchorhynchus claytoni, Belonolaimus longicaudatus, Meloidogyne graminis and Paratrichodorus minor were the most prevalent and abundant species on golf courses in both states. Twelve species were new records of plant parasitic nematodes in turfgrasses in both North Carolina and South Carolina. Further work, such as the development of molecular tools to quickly identify species of root-knot, will lead to a better understanding of these pests and their impact on golf course turfgrasses.
Many nematologists and pathol-ogists, based on previous work and their own experiences, recognize these and other nematodes as significant but often overlooked pests in golf turf. These pests are unseen root parasites, and their effects can mimic other causes of weak turf which likely leads to some delay in diagnosing their true nature as primary pathogens, and even misdiagnosis of the nature of the malady under investigation. In many states, the primary diagnostic lab is not coupled with nematode identification services. Nematode identification and enumeration requires different techniques and experiences than those typically employed in diagnostic labs for fungal pathogens. This means that the diagnostician should also be trained in nematology, be familiar with the symptoms they can induce and have an appreciation of the potential contribution of nematodes as pests. This is especially important in the South, where nematodes are recognized and known to be important. It’s also important that nematodes be considered in other states where heat and drought stress are more variable.

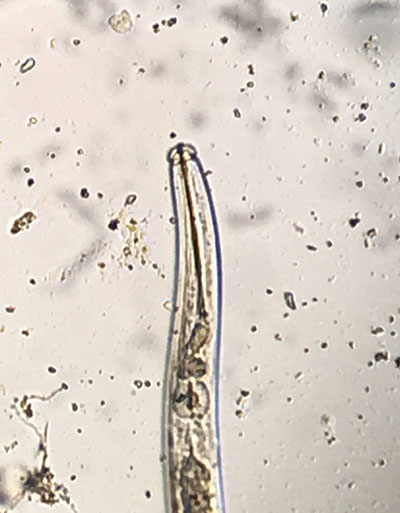
Figure 1 Belonolaimus longicaudatus (sting nematode) is a large nematode, reaching sizes of about 1/8-inch long when mature.
In southern states, arguably, the most important nematodes include sting, root-knot species and lance nematodes. Other species also cause significant damage themselves or as components of mixed populations if their counts are high enough. They can be a significant problem, but more credit is given them as significant pathogens when their counts considerably exceed threshold numbers. These might include nematodes such as stunt, stubby root, ring or even spiral nematodes.
Sting nematodes
Belonolaimus longicaudatus is a large nematode, reaching sizes of about 1/8-inch when mature (Figure 1). Size may particularly matter in the case of the stylet of this nematode, which is long and allows the nematode to feed deep within root tissues (Figure 2). The feeding habit of this nematode likely is why it causes damage even at low threshold numbers (most labs indicate a damage threshold of about 20 per 100 cc of soil). It feeds on root tips where the meristematic tissues are located, and thus stops root growth.
Feeding leads to a shallow, stunted root system, and wounds from the nematode feeding invite other soil organisms to colonize and further debilitate the host plants (Figure 3). Sting nematode is an ectoparasitic feeder — it’s body remains outside of the host tissues as it feeds, and it can move short distances to reach other roots for further feeding. As an ectoparasite, it’s presumed to be more susceptible to nematicides.
If there is anything good about a sting nematode (except being a fascinating organism), it is that it doesn’t survive in frozen soils for long periods and that it’s limited to soils of at least 80 percent sand. In the United States it is truly a southerner, although populations introduced into southern California have done well and are quarantined. Belonolaimus and related species also occur in turf in other parts of the world and are recognized as major pathogens. It’s obvious that sand-based putting greens are ideal habitats for sting and other nematodes. Besides being good habitats for the nematodes themselves, sand-based greens inherently are nutrient deficient and prone to drought stress. Therefore, root parasites such as nematodes are particularly important in sandy soils.
Root-knot nematodes
Nematodes in the genus Meloidogyne are the root-knot nematodes. At least five species have been identified in turf, including M. graminis, M. incognita, M. naasi, M. microtyla and M. marylandi. In bermudagrass turf, it appears M. graminis and M. marylandi are commonly identified. M. naasi and M. microtyla sometimes are identified in cool-season grasses as well as warm-season grasses. Root-knot nematodes are sedentary endoparasites. That is, they spend the majority of their lifecycles inside roots, except for newly hatched juveniles and males. Juveniles penetrate roots and set up feeding sites from where they no longer move (become sedentary), but instead induce giant cell formation from the host tissues. Giant cells of the host become metabolic sinks whose purpose is diverted from normal plant physiological processes to nutrition for the nematodes. In this way, root-knot nematodes are more stealthy parasites of their hosts.
Root-knot females within infected roots swell and become gourd-shaped when mature, producing eggs contained in a gelatinous egg sack (Figure 4). Egg sacks and mature female nematodes may break through the root cortex and become visible with a microscope. Results from control trials also have generally shown root-knot nematodes to be more difficult to manage than sting nematodes, probably because of their high reproductive capacity (multiple generations, high egg production) per season and the protection their bodies enjoy as endoparasites within roots. Root-knot nematodes are encountered in many soil textures, and are not limited to sandy soils. Their damage potential, however, likely is higher in sandy soils because of the inherent higher risk of drought and nutrient deficiency stresses.
Lance nematodes
Lance nematodes belong in the genus Hoplolaimus. The most commonly encountered lance nematode in turf is H. galeatus, although other species such as H. stephanus have been recognized recently. More research is needed in specific identification of lance nematode populations in turf, as the morphological differences among some species are not obvious.
Lance nematodes are migratory endoparasites in their feeding habits. They move inside and outside roots and feed seemingly indiscriminately on roots, not necessarily targeting specific regions of roots like sting or stubby root nematodes. A study in Kansas revealed that juvenile lance nematodes were most abundant within the roots, while more adults were encountered outside the root system. Lance nematodes have a high reproductive capacity, and in some locations counts easily can exceed several thousand per 100 cc of soil. Lance nematodes generally have relatively high damage thresholds, particularly in northern states in cool-season grasses, where environmental stress is less severe than in the South.
Damage thresholds
Plant parasitic nematodes are obligate parasites of their host plants. They must feed on these hosts in order to thrive, survive and reproduce. In most cases, we see separate sexes within a species of nematode, with males and females. Reproduction typically is by cross fertilization, which results in egg production by females. Development of a nematode from egg to adult is about a month. During this time, the nematode feeds and grows. It also sheds its chitin-based body wall several times to accommodate the increases in size as the nematode grows to adult and becomes sexually mature. Some nematodes, such as the root-knot nematodes, lay many eggs and their populations can reach high numbers in favorable conditions. Others, like sting nematodes, may not lay as many eggs and populations may not be nearly as high as those with high reproductive capacities. Nevertheless, some of these, like sting in particular, cause significantly more damage per individual nematode.
These biological phenomena and observations of damage, coupled with experimental evidence, have provided published “damage thresholds.” These are numbers of nematodes of a species per volume of soil or roots, and compare the potential of particular nematodes to cause meaningful damage. Generally, they are reported as numbers of individuals per 100 cc or per 500 cc of soil. Thresholds are guidelines in creating a diagnosis of particular nematodes in a particular problem.
As general guidelines, the numbers show how different nematodes compare relative to their pathogenicity or ability to cause damage. In some cases, thresholds are being refined to include different species, such as stubby root nematodes in the genus Trichodorus versus those in the genus Paratrichodorus (now Nanidorus). In Florida, Trichodorus obtusus has a lower threshold number than does Paratrichodorus on specific turfgrass species. Trichodorus obtusus has been found in North Carolina and South Carolina, so thresholds in the near future likely will be adjusted in these states to include this more damaging stubby root nematode.
Sampling
For nematode damage thresholds to have their greatest value, some thought into sampling methods is needed for sampling of soil and root systems. One obvious factor is that nematodes are going to be most abundant where roots are present. Rooting in golf course greens generally extends to about 4 inches, but may be shallower if a particular nematode problem is present. This is the depth of sampling generally recommended, but some nematodes — like root-knot — seem to be concentrated much more densely in the upper 1 to 2 inches, so stratified sampling may be appropriate.

Figure 5 A putting green with nematode damaged turf outlined in orange. Sample the margins of the damaged area where there is still green turf and symptoms are showing.
Also, nematodes are not uniformly distributed throughout a green, tee or fairway. Rather, they typically cluster in “hot spots” that may correspond to more favorable soil texture (e.g. sandy areas). This means that sampling for diagnostic purposes (to answer the question of whether nematodes are responsible for the symptoms expressed) is different than more routine or systematic sampling that might be done to determine an average population in an area, such as a research plot.
Diagnostic samples should be taken at the margin of damaged patches or areas. The objective is to find damaging species at high levels, if they exist. Do not sample dead or extremely thin turf, as nematodes are more likely to be at low populations where their food base is scarce. Rather, sample the margins, where there is still turf but where the turf is showing symptoms (Figure 5). Most labs prefer multiple cores of 1-inch diameter taken at the recommended depth and bulked to provide about 500 cc of soil. The lab will mix and subsample from the composite sample and provide counts of encountered nematode species. If the purpose is to monitor nematode populations in a turf stand, systematic sampling typically is recommended. Areas may be divided into plots (e.g. treated with a nematicide versus not treated), with multiple cores taken from each area in a zig-zag pattern to provide a good average count. We pay no attention to symptomatic turf in this method, so hot spots as well as healthier turf may be sampled.
Nematode populations change over time. Generally, populations are highest when plant growth, especially root growth, is favored, and may be lower when plant growth is slower or dormant. For bermudagrass in the southern Transition Zone for instance, root growth is abundant just as bermudagrass is moving out of winter dormancy in spring (Figure 6). Soil temperatures are likely still cool, 50 to 60 degrees F, and top growth is slow or almost imperceptible. Nematodes, if present, already may be feeding on these young roots. The same phenomenon occurs in fall when soil temperatures dip again as bermudagrass approaches dormancy. New roots appear in September and October but may also form in November and early December, when frosts have induced semi-dormancy and top growth is suppressed. Typically, a good time to sample for high levels of nematodes in this region would be April/May or September/October. Sampling for sting nematode in mid- or late summer in this region and even in Florida may show lowered populations as soil temperatures are high, and sting nematodes may move deeper in soil profiles and beyond the typical sampling depths. In winter months, counts may be lower because of nematode mortality.
Nematode management
Like most pests, options for management include cultural, biological and chemical methods. IPM strategies typically provide the most practical and consistent results. Even in southern regions where nematode induced stress is high, the most practical management is cultural and depends on the knowledge and skill of superintendents. These practices include spoon feeding nutrients, hand-watering greens, avoiding stressful verticutting/aerification practices when the root system is so damaged that turf can heave, and other practices. These practices are labor and knowledge intensive. In some cases, even when nematode damage is severe, a putting surface can be maintained at an acceptable quality for short periods. The problem is that the risk is high for damage to exceed acceptable levels if the environmental stress becomes too high.
Biological controls include natural products that may suppress nematodes, such as certain extracts of sesame, neem or mustard-based products that release glucosinolate metabolites that are nematicidal. Some biological products are based on microorganisms that are antagonistic to nematodes and perhaps exclude them from their feeding sites or produce metabolites that harm nematodes.
One such product is the bacterium Bacillus firmus strain I-1582, the active ingredient in Nortica (Bayer). It’s thought that this bacterium is antagonistic to certain nematodes, but it also may promote plant growth, thereby effectively avoiding some of the stress induced by nematode-compromised root systems. Other bacteria can be parasites of nematodes, such as species of Pasteuria. These are naturally occurring parasites, with strains that are more or less specific to their particular hosts (e.g. P. usgae parasitizes sting nematode but not root-knot or lance). As of this writing, Pasteuria bacteria are not available in a product, but there is hope that an effective biological control of certain damaging nematodes may be developed.
Where nematode damage is chronic and frequently severe, the use of chemical nematicides has provided the best method for suppressing populations and allowing turf to tolerate the stressful months adequately.
Three new nematicides were introduced in 2016 and 2017, including fluensulfone, the active ingredient in Nimitz Pro G, marketed by Quali-Pro. Also, fluopyram, the active ingredient in Bayer’s Indemnify, is registered and was sold as of late 2016.
Finally, abamectin, the active ingredient in DivaNem, developed and marketed by Syngenta, is available and replaces the Avid 0.15EC formulation of abamectin, which had previously been available under 24c labels in certain states. All of these materials are much safer than the old pesticides used for nematode control. As with any material for control, we are presently learning more about timing, placement, rates and effectiveness for nematodes.
Strategies that develop over time likely will include more than a single product, as none of these materials seems to be highly effective against the “big three” nematode species in the South.
Bruce Martin, Ph.D., is a turfgrass pathologist at Clemson University. You can reach him at sbmrtn@clemson.edu for more information.
All photos by Bruce Martin
References
Christie, J. R., Good, J. M., and Walter, G. C. 1954. Nematodes associated with injury to turf. Proc. Soil Sci. Soc. Florida 14: 167-169.
Crow, W.T., and Welch, J. K. 2004. Root reductions of St. Augustinegrass (Stenotaphrum secundatum) and hybrid bermudagrass (Cynodon dactylon x transvaalensis) induced by Trichodorus obtusus and Paratrichodorus minor. Nematropica 34:31-37.
Dickerson, O., Blake, J. H., and Lewis, S. A. 2000. Nematode guidelines for South Carolina. Clemson S.C., USA; Clemson Cooperative Extension.
Peacock, Charles H. 1989. Turf nematodes: dealing with an old problem. Conf. Proceedings: 60th International Golf Course Conference and Show, p. 73.
Settle, D. M.; Fry, J. D.; Milliken, G. A.; Tisserat, N. A.; Todd, T. C. 2007. Quantifying the effects of lance nematode parasitism in creeping bentgrass. Plant Disease. September. 91(9): p. 1170-1179.
Shaver, Bradly R., Martin, S. Bruce, Bridges, W.C., and Agudelo, Paula. 2015. Effects of Trichodorus obtusus on zoysiagrass and bermudagrass root weight and turf quality. Nematology 17:671-678.
Ye, Weimin, Zeng, Yongsan, and Kerns, James. 2015. Molecular characterization and diagnosis of root-knot nematodes (Meloidogyne spp.) from turfgrasses in North Carolina, USA. PloS ONE Nov. 24, 10 (11): p.e0143556 (1-16).
Zeng, Y., Ye, Weimin, Martin, S. Bruce, Martin, Matt, and Tredway, Lane. 2012. Diversity and occurrence of plant-parasitic nematodes associated with golf course turfgrasses in North and South Carolina, USA. Journal of Nematology 44:337-347.