Mowing down weevils

Figure 1 Time-lapse photography was used to capture annual bluegrass weevil (ABW) surface movement in growth chamber studies. Weevils were marked with a UV pen and placed in groups of five on a Poa annua plug. The black light illuminated the mark during dark periods. (Photo: Ben McGraw)
Many turfgrass managers in northeastern North America would rather forget the 2018 growing season. Record-breaking rainfall throughout the region affected most aspects of turfgrass management, including insect pest control. The annual bluegrass weevil (ABW), Listronotus maculicollis, is one insect pest that flourished in the wet weather and was particularly difficult to control with our standard arsenal of insecticides.
The weevil is challenging to manage even in a “normal” year, given that early larval instars are hidden within the plant. These “cryptic” stages are difficult to detect and nearly impossible to control with most — if not all — insecticides. This creates a need to manage the pest either preventively (targeting adults prior to egg laying) or curatively, as larvae emerge from the plant to feed externally on the crown. Both chemical management strategies have their advantages and disadvantages, with each requiring precision timing.

Figure 2A Two ABWs marked with a Sharpie Neon (bottom) and two unmarked weevils photographed without the filter system. (Photo: Charles Mazel)
Though many superintendents find it desirable to reduce populations preventively, only two chemical classes provide even moderate control of adults. The fear of creating a pyrethroid-resistant or possibly a multiple-insecticide-resistant population (one that is less sensitive to nonpyrethroid compounds as well) has many choosing to solely target larvae. Curative controls are more selective than broad-spectrum adulticides yet are relatively more expensive and require a detailed monitoring program to determine population structure.
Figure 2B The same weevils were photographed with the NIGHTSEA system as in Figure 2(A). The black light illuminated the mark during dark periods. (Photo: Charles Mazel)
Even with this information, spring 2018 curative applications required dodging heavy rainfall and saturated soils. We observed numerous product failures (including time-tested standards) in this year’s field trials, where applications were made in advance of heavy (greater than 1 inch) or prolonged rainfall. Clearly, relying solely on chemical insecticides to reduce ABW populations is a weak strategy, and a more integrated control approach is needed to manage the ABW in the future.
Mechanical removal of adults
Over the last four years, members of the Turfgrass Entomology Laboratory at The Pennsylvania State University have been investigating the impact that cultural controls, specifically mowing, nitrogen fertilization and irrigation, have on reducing ABW populations or damage severity. The project was initiated after reviewing superintendent responses to a 2016 ABW management survey.

Figure 3 The custom-made camera box photographed marked ABWs on top of the turfgrass canopy. The box housed the external flash (left) and camera (right) and provided consistency in light level and photo height throughout the experiment. (Photo: Ben McGraw)
It is uncommon to observe damage to putting surfaces maintained at or below 0.125 inch, though adjacent collars (0.25 inch and higher) may be severely affected. Superintendents reported much greater ABW incidence in collars than greens. Additionally, the percentage of greens experiencing turf loss increased linearly with increases in height of cut, suggesting that mowing may inhibit either ABW adult or larval persistence in these areas.
Greenhouse and field plot trials confirmed our suspicions, as 26 percent to 38 percent of adults were removed from the turfgrass canopy in a single mowing. Mowing height had a significant impact on adult removal, as the percent removed decreased at increasing mowing heights, with practically no removal at collar height of cut. The percentage of adults removed was encouraging given that the true impact is likely to be additive as greens are mowed five to seven days per week throughout the growing season.
However, superintendents most likely can’t rely on mowing alone as a stand-alone tactic, as we detected eggs in our lowest mowing height treatments (0.100 inch) that were capable of surviving to damaging stages. We need a better understanding of adult behavior and movement within the turfgrass canopy to identify conditions or periods when mowing can have an even greater impact on adult populations on putting surfaces and higher-mown areas (fairways, tees).
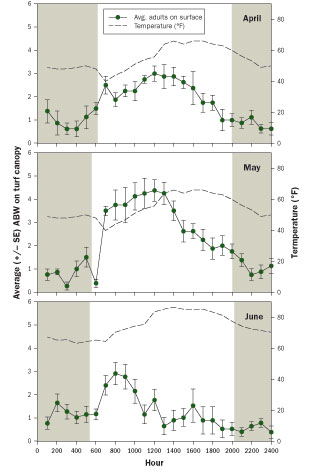
Figure 4 Effect of temperature and daylight on ABW canopy activity in three 24-hour observation periods. The shaded bars indicate periods of darkness. (Source: Ben McGraw)
Therefore, to optimize adult removal, we initiated a second study to understand when weevils were on top of the turfgrass canopy and to identify periods when mowing would be most effective.
Laboratory studies
Most animals exhibit diel patterns — an increase in activity with regard to time of day or photoperiod — that allow them to move, feed, escape predation or mate. Our efforts to improve ABW mechanical removal through mowing required a better understanding of ABW surface activity and the factors that influence movement. Our investigations sought to assess the effects of photoperiod (light/dark periods) and temperature on adult activity.
It had been anecdotally reported by researchers that ABWs are nocturnal, which was not surprising because many weevils become most active near dusk or in the middle of the night. However, making direct observations of ABW behavior is difficult because the weevil is relatively small (0.14 to 0.18 inch in length) and requires tracking movement in darkness. We initially observed weevil surface activity in growth chambers using time-lapse photography.
To overcome the challenge of observing movement through both light and dark phases, we marked weevils with a UV pen, which caused the weevil to fluoresce under a black light (Figure 1). This system allowed for near-continuous observations under precise light and temperature conditions. We found surface activity to be greatest between 59 degrees to 68 degrees F and equal light/dark phases, with few weevils active on the surface when temperatures were less than 50 degrees F. We detected no differences in surface movement with regard to time of day.
In a second experiment, we programmed incubators to exhibit different daytime and nighttime conditions (14 hours of light at 64 degrees F, 10 hours of darkness at 50 degrees F) such as those encountered in spring during the overwintering adult migration period. These conditions resulted in diurnal activity, with greatest activity around midday and little to no activity during the dark phase. However, when we reversed temperatures for light and dark phases (10 hours of light at 50 degrees F, 14 hours of darkness at 64 degrees F), we observed the opposite, with the greatest activity in the middle of the night and little to no activity during the light phase, suggesting that temperature, not photoperiod, had a greater impact on surface activity.
In-field studies
We attempted to observe adult activity in the field using the same system described for laboratory studies — marking insects with a UV mark, placing ABWs in cages with a UV light and capturing movement on a time-lapse camera. No matter how hard we tried to exclude outside insects (e.g., moths, flies) — including surrounding the study area with mesh cages — nocturnal insects somehow got in, attracted to the UV light and blocking the time-lapse images.
We solved our problem through a random encounter on the 2015 tradeshow floor of the Entomological Society of America’s annual meeting. We met Charlie Mazel, Ph.D., of NIGHTSEA, a developer of fluorescent camera systems. Mazel’s background is in underwater exploration and photography of corals, and his company develops solutions for viewing and photographing fluorescent images (https://www.nightsea.com/about-us/).
His solution involved marking weevils with a Sharpie Neon pen, then capturing an image with a digital single-lens reflex (DSLR) camera (Nikon D90), off-camera flash and light filters. The photographer places one filter on the camera’s external flash to produce a blue light flash. A yellow blocking filter is placed over the camera to exclude reflected blue light and transmit only the fluorescence, resulting in super-excitation of the marked weevil (Figure 2a-b). We used a custom-made photo box (12-inch width by 12-inch depth by 18-inch height) to hold the camera and flash over each observational arena and to block out sunlight (Figure 3). The camera and flash were threaded through holes in the lid of the box to ensure consistency of height between photos.
With the NIGHTSEA filter system, we could take a still image of the turfgrass canopy at regular intervals, and any insect on top of the canopy would fluoresce. The downside was that we could not continuously or remotely monitor movement, but instead we had to have someone physically move the camera box from observational arena to observational arena, taking photos every hour on the hour. This was done for 24-hour periods in April, May and June.
This novel mark-recapture system confirmed laboratory findings that adult activity was affected by temperature and not light. Adult weevil activity on top of the turfgrass canopy was greatest during the day and strongly correlated with temperature early in the season (April, May) (Figure 4). However, adult presence on the surface in early summer was greatest briefly after sunrise, then declined during the midmorning when temperatures exceeded 70 degrees F. The effect of temperature on surface activity in all months was best described by a model that predicts maximum adult surface activity between 57 degrees and 63 degrees F.
Strong temperature association
Our findings suggest that ABW adult surface activity is strongly associated with temperature. Therefore, scheduled mowing with the intent to remove adults may need alterations throughout the season. Although most operations must mow the putting surfaces first thing in the morning to prepare the course for the day and to stay ahead of golfers, performing a second “dry cut” to improve ball roll or possibly vacuuming surfaces when temperatures are between 57 degrees and 63 degrees F may provide improved adult control. Altering daily mowing routines to increase mechanical removal may not be necessary in the summer if early morning temperatures are in the optimal surface activity range we observed in the June field studies.
This study has other benefits to turfgrass managers than just mechanical control. Successful pest management depends on monitoring pest activity. We have observed that several of our monitoring techniques (e.g., vacuum sampling, soap flushing) are more successful when the insects are active. Timing regular scouting activities when temperatures are in the optimal temperature range minimizes the likelihood of missing an infestation.
This research also has implications for chemical management. The adulticides used to control ABW adults are short-residual contacts. We may improve contact insecticide efficacy by using our temperature model if less plant material will be between the insect and the toxin at application or if the insect moves through the residue. We will investigate this in the upcoming field season as a possible new cultural control method. It is my hope that these studies lead to simple, efficacious and cost-effective solutions that superintendents can implement along with or in lieu of current management plans.
Acknowledgements
This work was partially funded with a grant from the United States Golf Association (USGA), regional golf course superintendent groups (Central Pennsylvania Golf Course Superintendents Association, Connecticut Association of Golf Course Superintendents, Finger Lakes Association of Golf Course Superintendents, Greater Pittsburgh Golf Course Superintendents Association, Long Island Golf Course Superintendents Association, Mid-Atlantic Association of Golf Course Superintendents, Mountain and Valley Golf Course Superintendents Association, Northeastern Golf Course Superintendents Association, Northwest Pennsylvania Golf Course Superintendents Association, Old Dominion Golf Course Superintendents Association, Western New York Golf Course Superintendents Association) and support from the USDA National Institute of Food and Agriculture, Hatch project 1006804.
References
Czyzewski, B. D., and B. A. McGraw. 2017. Mowing height influences Listronotus maculicollis (Coleoptera: Curculionidae) oviposition behavior and mechanical removal from golf course putting greens, but not larval development. J. Econ. Entomol. 110: 2165 – 2171.
McGraw, B. A. and A. M. Koppenhöfer. 2017. A survey of regional trends in annual bluegrass weevil (Coleoptera: Curculionidae) management on golf courses in eastern North America. J. Integr. Pest Manage. 8: 2- 11.
Based on the article:
Czyzewski, B. D., and B. A. McGraw. In press. Detection of Listronotus maculicollis (Coleoptera: Curculionidae) turfgrass canopy activity with the use of a novel fluorescent marking system suggests opportunities for improved mechanical control. Environmental Entomology, https://doi.org/10.1093/ee/nvy156