How turfgrasses use urea-nitrogen
By Richard J. Hull, Ph.D., Haibo Liu, Ph.D. and N. Menchyk, Ph.D.
Nitrogen (N) is the most abundant mineral element in turfgrasses, comprising three to five percent of leaf dry weight. Consequently, N is the fertilizer nutrient applied to turf in a greater quantity than any other (two to five lbs per 1000 sq. ft. per year). Most of this N is applied to turf as urea, either as free urea, coated-urea granules or polymerized methylated urea.
Because of the perennial sod character of turf, most fertilizer N is applied directly to grass leaves in granular form or as an aqueous solution. Only during turf establishment is fertilizer spread directly on the soil. While most crops utilize less than 50 percent of fertilizer nitrogen (Witte, 2011), turfgrasses do much better, often recovering 85 to 90 percent (Hull & Liu, 2005).
Even with this relatively good performance, N recovery by turfgrasses may still be improved. Since urea is the major N source applied to turf, its absorption and assimilation will be critical factors in working for improved N use efficiency. Recent research has discovered some aspects of the uptake and metabolism of urea-N that may be exploited by turf managers to reduce their N use and prevent its off-site movement.
How urea-N is absorbed by turfgrasses
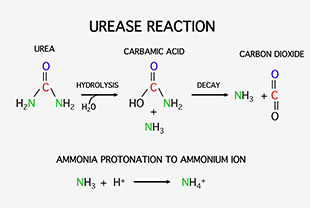
It has long been assumed that urea-N is not absorbed directly by plant roots but rather as ammonium (NH4+) or nitrate (NO3–) and by leaves as ammonia (NH3) or NH4+. Urea is not stable in the soil or on leaf surfaces due to the ubiquitous presence of the enzyme urease that hydrolyzes urea to carbamic acid and one free ammonia. The unstable carbamic acid then spontaneously decays to another ammonia and a carbon dioxide (CO2) molecule (Fig. 1). Both NH3 and CO2 are gases that would diffuse into the atmosphere and be lost were it not for their high solubility in water. CO2 can combine with water to form carbonic acid that under slightly acid pH conditions, dissociates to a bicarbonate anion and a free H+.
CO2 + H2OH2CO3HCO3– + H+
On the other hand, in an acid solution, NH3 will acquire a H+ (become protonated) to form the stable ammonium cation (NH4+) (Fig. 1). The pKa for NH3 protonation is 7.2 (the solution pH at which the concentrations of NH3 and NH4+ will be equal). Therefore, if the soil solution or the liquid on a leaf surface is more alkaline than 7.2, much nitrogen from hydrolyzed urea will remain in the gaseous NH3 form and readily defuse from solution into the atmosphere.
Fortunately, leaf surfaces and soil solutions are generally acid, so nitrogen derived from urea fertilizers will be maintained in the stable NH4+ form. In that form, nitrogen can enter plant cells via cation transporters and become assimilated into amino acids and eventually into nucleic acids, proteins, chlorophyll and other N-containing molecules.
However, in the soil, NH4+ will be oxidized rapidly to nitrate (NO3–) by nitrifying bacteria and in that form will be actively absorbed into root cells via specific NO3– transporter proteins imbedded in the plasma membranes of most plant cells. Nitrate ions are not subject to volatilization from the soil solution but they can leach in rain or irrigation water through the root zone and be lost to the water table. Thus, urea applied to the soil should be treated much as inorganic nitrogen materials, with respect to leaching and potential ground water contamination.
Urea is a natural chemical in most living things. It is formed during biosynthesis of the essential amino acid arginine and likely during the normal metabolism of nucleic acids (Hull 2003; Witte 2011). Urea can be regarded as a waste product and as such must be eliminated from the organism. This is no problem for animals that have a circulatory system. For plants, such wastes pose more of a problem.
Normally plant byproduct wastes are deposited in cellular vacuoles, where they are excluded from the sensitive metabolic machinery within the cytoplasm and where some recycling may occur. However, waste products from major metabolic pathways could be made in quantities large enough to overwhelm vacuolar sequestration and contribute to nutrient inefficiency especially when they are N-rich chemicals like urea. Since most plants are normally N-limited and could not tolerate such inefficiency, they have evolved a method for metabolizing urea and recycling its N. Urease is at the core of urea-N recovery.
The presence of the urease enzyme in most plant cells permits urea-N to be efficiently returned to the plant’s N assimilatory pathway and reutilized for the synthesis of essential N-containing compounds. The presence of urease makes plants, algae and many bacteria among the most N-efficient life-forms on earth. If a plant lacks the urease enzyme, urea can accumulate to toxic concentrations and eventually cause plant death. This occurs in mutant plants that fail to synthesize functional urease or when Nickel (Ni), an essential metallic component of urease, is deficient.
Nickel is the latest mineral element to be added to the list of essential nutrient elements in plants. When grasses are grown in solution cultures lacking Ni, severe leaf tip burn is observed resulting from an excessive accumulation of urea in this oldest part of a grass leaf (Hull, 2003). Because Ni is required in such small amounts (~0.1 ppm in dry leaf tissue) and grass leaves normally contain at least three to five times that amount, Ni deficiency has never been observed in the field (Menchyk et al. 2013).
Even so, Ni may have a role to play in the use of urea fertilizers applied to turf. We will get back to that later.
Can urea be absorbed by plants as an intact molecule?
Because urease-endowed organisms are so abundant in nature, it has been assumed that urea-N applied as fertilizer is readily hydrolyzed and absorbed by plants primarily as NH4+. While this is generally true, recent evidence indicates it may not be the whole story (Witte 2011). For example, plants can be grown in sterile solution culture with urea as the only N source. Such plants fail to grow as well as plants receiving a mixture of NH4+ and NO3– but they still grow reasonably well, indicating that intact urea must be entering root cells.
In addition, plants have been shown to possess dedicated high affinity urea transporter proteins in their cell membranes that permit urea uptake and distribution throughout the plant. Once inside plant cells, urea is readily hydrolyzed by their abundant urease or loaded into vacuoles by urea transporters that are also present in their tonoplasts (membrane enclosing a vacuole). The induction of these urea transporters appears to be stimulated by external urea and suppressed by the presence of other soluble N sources, e.g. NO3–, NH4+ and amino acids.
Urea transporters are most abundant in N-starved plants, especially when urea is introduced to their external environment and when plants are provided no N-source except urea.
The urea transporters discussed above facilitate secondarily active urea uptake. That is, urea crosses a membrane accompanied by a proton (H+) in a co-transport process. During normal root growth, the surface (epidermal) cells pump H+s through their plasma membrane from the cell’s interior into the external cell walls via a H+-transporting ATPase (H+ pump).
Put more simply, for each two H+s pumped out of a cell, an ATP molecule is expended. (ATP + H2OADP + H2PO4– + 2H+).
Since ATP is the chemical energy currency of metabolism, H+ pumping is an energy expending process that is driven by ATP hydrolysis. As this process continues, the H+ content within the cell walls increases (becomes more acid) relative to the cell interior that remains the same or actually becomes less acid. This pH gradient across the plasma membrane constitutes an energy gradient equivalent to the energy lost by ATP when it gives up a phosphate and becomes ADP.
How is this H+ gradient used to pump urea into a plant cell? When urea is introduced as an N-fertilizer, it diffuses into the walls of root or leaf epidermal cells where it activates the synthesis of urea transport proteins in their plasma membranes. These transport proteins have aqueous pores through which H+s can move from the cell walls back into the cell’s interior. The structure and chemistry of the pores is such that an H+ can pass through the pore only if accompanied by a urea molecule. Since the H+ concentration in the cell wall is greater than it is within the cell interior, influx of H+ is energetically favored. Urea will always be transported inward because the influx of H+, its transport partner, is an energetically favored process.
There is recent evidence that urea may also enter plant cells and move across internal cell membranes via aquaporins, the membrane channels by which water enters cells (Witte 2011). Like water, urea is a small, uncharged molecule that probably would not be excluded from moving passively with water through aquaporin channels. Whether urea can enter cells as an intact molecule by active or passive mechanisms is less important than the emerging idea that urea-N can be absorbed by roots and leaves without first being released as free NH3.
Managing urea-N uptake by turfgrasses
To a turf manager, all this may seem pretty academic and of little practical value toward keeping grass green and healthy by maintaining an adequate N supply. Such a judgment may be premature when the vulnerability of NH3-N to volatility losses is considered. We know that as much as 30 percent of urea-N applied to turf can be lost to the atmosphere unless it is watered into the thatch/soil by irrigation immediately following application (Hull and Liu 2005). This precaution fails to work very well if the soil is even slightly alkaline or the irrigation water has a pH greater than seven. Even if the soil is acid enough to convert NH3 to NH4+ ion, it will be readily oxidized to NO3– that may be subject to leaching loss.
Foliar applied urea initially comes in contact with leaf surfaces that may be populated by enough urease-containing bacteria to cause most of the urea-N to be released as free NH3. However, leaf surfaces are generally acidic, so urea-derived NH3 will likely become NH4+ ions in the spray solution retained on leaf surfaces. These NH4+ ions can penetrate the leaf cuticle and become absorbed by epidermal cells just as other nutrient ions are during foliar fertilization. If the spray solution dries on the leaf surface, any remaining NH3 will be lost to the atmosphere.
Does an understanding of urea-N absorption and metabolism suggest ways of making urea use more efficient?
Recent research by our Clemson team (Menchyk et al. 2013) indicates that the efficiency of urea-N use by turf can be optimized by coordinating its application with management of the micronutrient, Nickel (Ni). They worked with two warm-season grass species, TifEagle ultradwarf bermudagrass (Cynodon dactylon x C. transvaalensis) and Diamond Zoysiagrass (Zoysia matrella). Plugs of these grasses were grown in greenhouse conditions with the nutrient solution supplemented with three levels of Ni (0, 200 & 400 µg Ni per liter). Nitrogen was supplied exclusively through weekly foliar applications of urea at a rate equivalent to 0.2 lbs. N per 1,000 sq. ft. (1.8 lbs. N per 1,000 sq. ft. during the nine-week duration of the experiment).
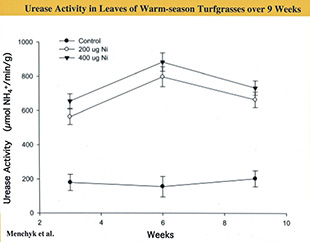
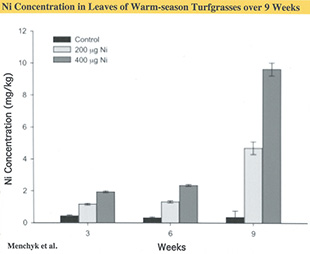
The addition of Ni to the nutrient solution substantially increased the leaf Ni content at a rate that increased throughout the nine weeks of the experiment (Fig. 2). This resulted in a dramatic stimulation of leaf urease activity that, unlike the Ni content, leveled off after six weeks (Fig. 3). The amino acid content of leaves increased steadily following a pattern very similar to Ni increase.
Not surprisingly, these changes contributed to greater leaf growth in both grasses. However, with this stimulated N metabolism occurring, the total leaf N concentration actually declined. Others have observed this and attributed it to a dilution of plant nitrogen by increased leaf growth. In this experiment, all N was being introduced from foliar applied urea. As growth was stimulated throughout the plant, its demand for N would increase, causing a drain in leaf N.
This study reveals ways by which Ni manipulation, as a way of regulating urease activity, might become a management tool for increasing N use efficiency. If urea-N can enter grass roots or leaves as intact urea molecules, and by applying Ni, internal urease activity can be stimulated, the concentration gradient of urea across the plasma membranes of root and leaf epidermal cells can be increased. Such a steepened urea gradient would stimulate urea influx, especially via aquaporin-like membrane channels.
This probably has less potential benefit for soil-applied urea since the natural sources of urease are so abundant there, that urea would have a short half-life in most soils. However, if urease inhibitors are used in conjunction with urea absorption stimulation techniques, direct urea absorption by roots could be enhanced with less urea-N lost via NO3– leaching.
The greatest potential for increased urea-N recovery by turf may be in the foliar application of urea. If Ni levels in turfgrasses can be increased via granular fertilizer application, fertigation or injection techniques, thereby increasing urease activity within leaf cells, foliar-applied urea may be more efficiently absorbed. Applying foliar urea in the evening at concentrations that have little burn potential and delaying irrigation until morning could significantly increase the opportunity for intact urea adsorption. Including a surfactant in the urea solution would increase leaf coverage and also favor absorption. Of course, using mildly acidic water will keep any free NH3 in the NH4+ form that will be more readily absorbed.
These are suggestions. They have a reasonably sound scientific basis for working. In any event, they can do no harm, and are worth trying.
Richard J. Hull, Ph.D., is a professor emeritus of plant science at the University of Rhode Island and adjunct professor of horticulture at Clemson University. Haibo Liu, Ph.D., professor of turfgrass science and N. Menchyk, Ph.D. are at Clemson University. Dick Hull can be contacted at rjhull34@yahoo.com for more information.
References
Hull, R.J. 2003. How do turfgrasses use nickel. Golfdom (TurfGrass Trends) 59(12):52,
Hull, R.J. and H. Liu. 2005. Turfgrass nitrogen: physiology and environmental impacts. International Turfgrass Society Research Journal 10:962-975.
Menchyk, N., D.G. Bielenberg, S.B. Martin, H. Luo and H. Liu, 2013. Supplemental nickel applications and foliar urea fertility on two warm-season turfgrass species under salinity stress. Crop Science (In press).
Witte, Claus-Peter, 2011, Urea metabolism in plants. Plant Sciences 180:431-438.