A close look at the impact of golf courses on pond ecosystems
Golf courses in the United States play a significant role in maintaining and enhancing local biodiversity — mainly when the adjacent landscape is urban or agricultural (1). Managed areas that harbor native biodiversity are crucial for supplying source populations to adjacent areas and maintaining ecological processes and ecosystem integrity.
Turfgrass ecosystems constitute a significant component of land use in suburban and urban areas. Commitment to sustainable management has led to these systems providing substantial acreage for maintaining native biodiversity (2,3). Still, these systems are also intensively managed compared to other land-use types (4).
Management practices
Turfgrass management practices typically involve chemicals affecting water quality, aesthetics and native beneficial organisms via runoff into surface waters. The effect of these chemicals is a function of their mode of action, loading rates and half-life (5). The use of pesticides and fertilizers, in particular, can have non-target effects on adjacent ecosystems (6,7). Pesticide impacts on amphibians have harmful effects on survival, development and growth (8,9,10) and concentrations below lethal exposure can create several sublethal effects, including malformities (11).
Nutrient additions via fertilizers can sometimes increase nitrogen and phosphorus in ponds located within turfgrass ecosystems (12). Additions of nitrogen and phosphorus in agriculture have caused several issues; still, concentrations are far less for golf courses — indicating turfgrass management may buffer against the effects of nutrient additions (13). Nutrient additions at environmentally relevant concentrations can have sublethal effects on biofilms (e.g., algae), invertebrates and amphibians in ponds (14,15,16).
Therefore, understanding the impact of turfgrass management on the ecosystem benefits of golf courses will provide insights into their value in the face of continued global change.
The objectives of this research were to survey water quality and biotic communities of lentic (herein referred to as ponds) ecosystems across 25 golf courses and then use data from survey efforts to examine whether additions of a pesticide and nutrients (e.g., nitrogen and phosphorus) have measurable impacts on adjacent pond water quality and ecosystem communities.

(Figure 1) Randomized array of mesocosms to examine the influence of azoxystrobin, nitrogen and phosphorus on pond biotic communities. (Photo: Joseph Milanovich, Ph.D.)
Biological and water quality surveys
We sampled water quality and vegetation twice annually (April and August 2017) at a single pond ecosystem adjacent to a fairway on each of the 25 golf courses in the Chicago metropolitan area. During each sampling period, we measured water-column dissolved oxygen (milligram/liter), nitrate (milligram/liter), ammonia (milligram/liter), conductivity (microsecond/centimeter) and algal abundance (blue-green (phycocyanin) and green (chlorophyll a); microgram/liter) using a YSI EXO2 Sonde. After digestion by acid-persulfate oxidation, we measured the water column’s total phosphorus by analyzing filtered water samples using the molybdate blue-ascorbic acid reaction.
Our team quantitatively sampled zooplankton and macroinvertebrate diversity and abundance with a 20 centimeters diameter 80-micrometer mesh plankton net attached to a 74-micrometer mesh bucket and a D-frame dip net (500-micrometer mesh), respectively. Plankton and D-frame dip nets were swept five times across a 0.3-meter (linear) area. We preserved all samples in 70 percent ethanol and identified to the lowest possible taxonomic level (20).
Amphibian diversity, abundance and percentage of malformities were quantitatively sampled using five pipe samples per pond on five dates between March and August 2017. The pipe sampler consists of a 52-by-27-centimeter galvanized steel trash can with the bottom removed and the perimeter smoothed to not harm the organisms. To take each sample, we quietly approached the sampling site and quickly pushed the can into the substratum of the wetland. We used nets (23-by-15 centimeters) to remove all animals within the can and water column until we took 10 consecutive sweeps without capturing an individual.
Mesocosm experiments
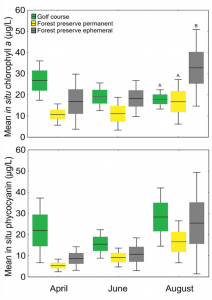
(Figure 2) Box plots (line = mean, box = SE and whiskers = 95 percent confidence interval) of in situ chlorophyll a and phycocyanin concentrations within sites across months. Different upper case letters suggest statistical significance (p≤0.05) within months using one-way ANOVAs and Tukey multiple comparison tests.
In the second and third years, we utilized a randomized array of mesocosms to examine the influence of pesticides and nutrients found in golf course water samples. Mesocosms are small tanks (Beckett Corp.; approximately 30-centimeter water depth and 99 centimeters diameter) stocked with 120 liters of tap water and 150 grams of hybrid cattail (Typha x glauca) to provide a consistent food source. We inoculated each mesocosm with 1 liter of pond water from the locations where we collected egg masses and invertebrates to provide colonization of biofilms (algae) and zooplankton (Figure 1).
Results and discussion
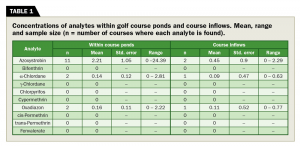
Water quality/chemistry and algae: We examined concentrations of 10 analytes (Table 1) within each of the 25 golf course ponds and seven accessible course inflows (e.g., courses with accessible wells or naturally filled ponds) in April and August 2017. Azoxystrobin, α-chlordane and oxadiazon were the only compounds above the detectable limit in any of the 25 courses or course inflows in August, and no detections occurred in the April samples. Nearly 30 percent of course inflows we examined (3/7) had detectable compounds that likely contributed to the concentrations found in course ponds (Table 1).
Concentrations of chlorophyll a were only significantly different between ephemeral (i.e., temporary) ponds and the forest preserve/golf course ponds in August (Figure 2), whereas concentrations of blue/green algae (phycocyanin) were not measurably different between any pond type.
Water quality variables taken with a YSI multi-probe meter show significant differences between golf courses and temporary wetlands but not between golf courses and forest preserve ponds (data not shown). Water chemistry values (i.e., nitrate, ammonium and phosphorus) show temporary wetlands have higher nitrogen and phosphorus levels than the golf course and forest preserve ponds in April and June (Figure 3). This data suggests the water quality and chemistry of golf course ecosystems are similar to adjacent permanent, fish-containing forested ponds in managed forest preserves (Piacente et al., 2020).
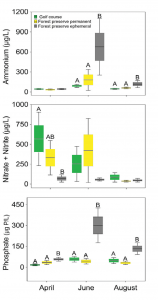
(Figure 3) Box plots (line = mean, box = SE and whiskers = 95 percent confidence interval) of phosphorus, ammonium and nitrate and nitrite values within sites and across months. Different upper-case letters indicate statistical significance (p ≤ 0.05) within months using the Tukey HSD test.
Biotic assessment: We collected 495 macroinvertebrates and 495 microinvertebrate samples from April to August 2017 (3 or 5 each month/site) to quantify micro (zooplankton) and macroinvertebrate diversity and density. To date, our results show species diversity of macroinvertebrates on golf course pond ecosystems from April to August 2017 using Shannon’s diversity index. (The lower the value of H, the lower the diversity). A value of H = 0 indicates a community that only has one species.
Our results show diversity on golf courses (H=1.38) is higher but similar to permanent, fish-filled (H=1.36) and lower than temporary, fishless adjacent ecosystems (H=1.41); however, these represent an incomplete data set of 20 golf courses, 13 temporary and 11 permanent, fish-filled sites (Figure 4). Our results indicate microinvertebrate diversity between April to August 2017 was variable but was more similar across pond ecosystem types than macroinvertebrates (data not shown).
First experimental mesocosms: We designed a mesocosm experiment to investigate the influence of azoxystrobin fungicides, nitrogen (N) and phosphorus (P) additions on aquatic ecosystems. In the spring/summer of 2018, we implemented a randomized experiment with nine treatments (see Table 2 for mesocosm treatments and concentrations). We replicated each treatment with 4 to 6 mesocosms using organisms from three trophic levels: amphibians (American toads and leopard frogs), dragonfly nymphs (Pantala and Leucorrhinia) and biofilms (algae) for a total of 120 mesocosm units.
Our results suggest chlorophyll a and phycocyanin concentrations did not differ across treatments. However, American toads raised in low concentrations of azoxystrobin were smaller than control or high-concentration azoxystrobin mesocosms (Figure 5). We believe this may result from a decreased, though not significant, amount of food resources. We also found that high nutrient levels, particularly N and N+P, resulted in larger toads at metamorphosis, but low phosphorus concentrations led to smaller toads (data not shown).
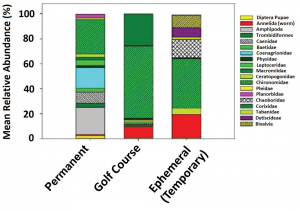
(Figure 4) Mean relative abundance of macroinvertebrate families in a permanent, fish-filled forest preserve or golf course ponds and ephemeral wetlands in August 2017. We presented only taxa representing more than 1 percent of relative abundance across all sites.
Second experimental mesocosms: In the spring/summer of 2019, we implemented a randomized experiment with 15 treatments (see Table 2 for mesocosm treatment concentrations). We replicated each treatment with four mesocosms using organisms from two trophic levels: amphibians (American toads) and biofilms for a total of 120 mesocosm units. Our results suggest American toads raised in low or high concentrations of azoxystrobin or N, P and N and P reached metamorphosis significantly faster than control animals and were significantly larger and no measurable difference in survival (Figure 6).
In addition, we examined the influence of N, P, N and P and azoxystrobin concentrations on dragonfly nymphs (Pantala and Leucorrhinia) survival and mass across 30 days using 10 liter plastic containers. We fed dragonflies black worms after being exposed to treated water. We changed the water with control water after the initial treatment inoculation. We found all nymphs survived the full 30 days in single combination doses and although mass varied across the treatments, it was not significantly different. In combined treatments, variation existed for mass and survival; however, the results were insignificant.
This article was adapted from:
Milanovich, Joseph; Berg, Martin. 2019. Examining the response of golf course lentic ecosystems to insecticide and nutrient additions using survey and experimental approaches. Turfgrass and Environmental Research Program: 2019 Research Summaries. p. 356-373.
Piacente, Jennifer N., Joseph R. Milanovich, Martin B. Berg, Timothy J. Hoellein, Andrés G. Muñoz, Armand A. Canna and Isabella S. Lentinia. 2020. Characterizing lentic habitats in golf courses and adjacent green spaces: water quality, water chemistry, pesticide concentrations and algal concentrations. Journal of Freshwater Ecology. 35(1):507-522.
References
- Colding J, Folke C. The role of golf courses in biodiversity conservation and ecosystem management. Ecosystems. 2009; 12:191-206.
- Semlitsch RD, et al. Using golf courses to bolster amphibian communities. USGA Green Section Record. 2007; 45:7-11.
- Tanner R, Gange A. Effects of golf courses on local biodiversity. Landscp Urb Plan. 2005; 71:137-146.
- Smith A, Bridges D. Movement of certain herbicides following application to simulated golf course greens and fairways. Crop Sci. 1996; 36:1439-1445.
- Christians N (2011) Fundamentals of turfgrass management: John Wiley & Sons.
- Peck DC. Comparative impacts of white grub (Coleoptera: Scarabaeidae) control products on the abundance of non-target soil-active arthropods in turfgrass. Pedobiologia. 2009; 52:287-299.
- McDonald DK, David K. Ecologically sound lawn care for the Pacific Northwest findings from the scientific literature and recommendations from turf professionals. 1999.
- Boone MD, et al. Suitability of golf course ponds for amphibian metamorphosis when bullfrogs are removed. Conserv Bio. 2008; 22:172-179.
- Relyea RA. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl. 2005; 15:618-627.
- Sparling DW, Fellers GM. Toxicity of two insecticides to California, USA, anurans and its relevance to declining amphibian populations. Environ Toxicol Chem. 2009; 28:1696-1703.
- Sparling DW, et al. In situ effects of pesticides on amphibians in the Sierra Nevada. Ecotoxicology. 2015; 24:262-278.
- Stier JC, et al. (2013) Turfgrass: Biology, Use and Management: Amer. Soc. Agron.
- Petrovic AM, Easton ZM. The role of turfgrass management in the water quality of urban environments. Int Turfgrass Soc Res J. 2005; 10:55-69.
- Griffis-Kyle KL. Sublethal effects of nitrite on eastern tiger salamander (Ambystoma tigrinum tigrinum) and wood frog (Rana sylvatica) embryos and larvae: implications for field populations. Aqt Ecol. 2007; 41:119-127.
- Fairchild GW, Lowe RL. Artificial substrates which release nutrients: effects on periphyton and invertebrate succession. Hydrobiologia. 1984; 114:29-37.
- Declerck SA, et al. Effects of nutrient additions and macrophyte composition on invertebrate community assembly and diversity in experimental ponds. Basic Appl Ecol. 2011; 12:466-475.